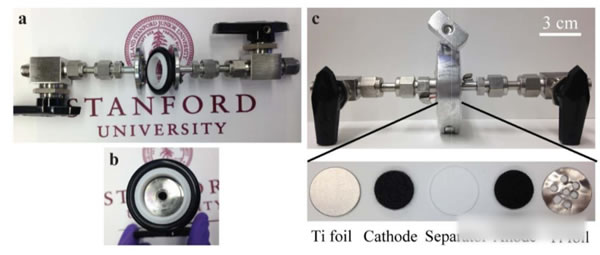
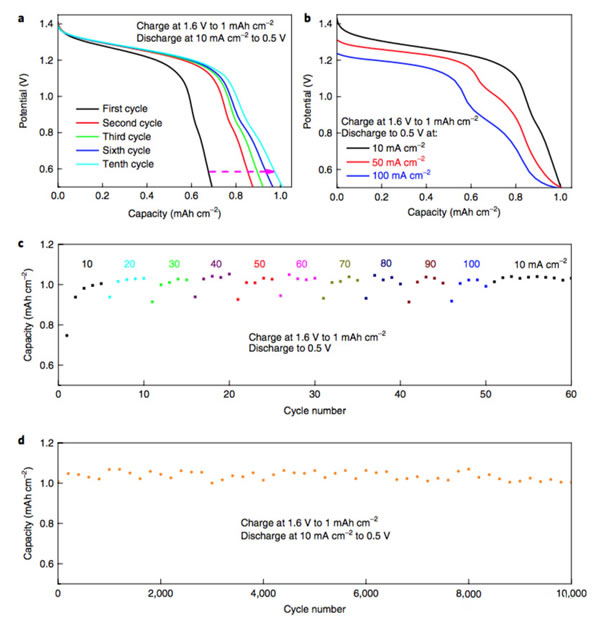
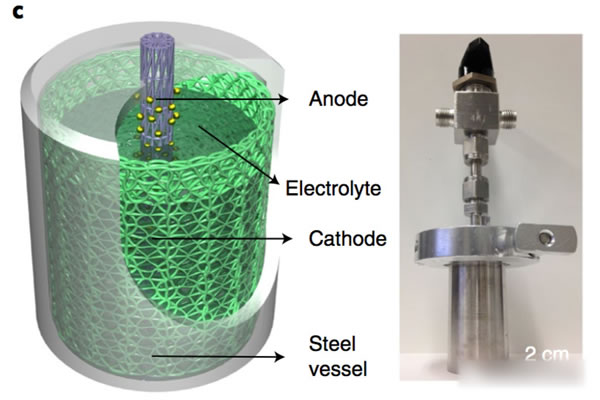
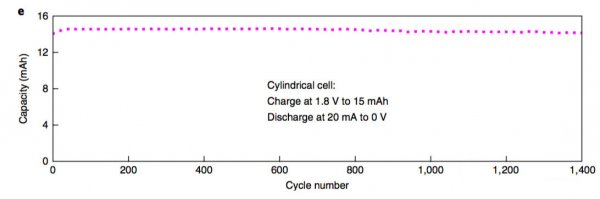
Lithium-ion batteries have the advantages of high voltage (3.7V), high specific energy (200Wh/kg or more), and long life, and are currently the first choice for electric vehicles. However, with the dramatic increase in the demand for power batteries, they have also boosted the correlation. The prices of upstream raw material products and lithium-ion battery-related raw materials, such as lithium, cobalt, nickel, etc., have all increased significantly in 2017. In particular, the price increases of lithium and cobalt materials can be described as crazy. . Under the dual pressure of high production costs of power batteries and cost reductions from downstream vehicle manufacturers in the industrial chain, profit margins of power battery manufacturers are greatly squeezed. At the same time, under the fierce market competition, power battery manufacturers lack the pricing power. Therefore, it will be a very difficult year for the majority of power battery manufacturers in 2018. Enhancing the performance of the power battery and reducing the production cost is the key to the future development of the battery. Recently, Cui Wei and Wei Chen of Stanford University have jointly developed a new battery based on MnO2-H2. The battery uses an aqueous solution as the electrolyte. The working voltage reaches 1.3V. Through optimizing the actual specific capacity of the battery up to 139Wh/kg (theoretical specific capacity is about 174Wh/kg), its cycle life can reach more than 10000 times, and it has the advantage of low cost, so it is in the energy storage And power battery has a broad application prospects. The working principle of the positive electrode of Mn-H battery is the transition between soluble Mn2+ and solid MnO2. The negative electrode adopts the transition between H+ and H2. The electrolyte is a high concentration of MnSO4, unlike the traditional solid-state electrode. Both positive and negative electrode reaction products are soluble (as shown in the formula below). The structure of the Mn-H battery is shown in the figure below. The positive electrode adopts a carbon fiber felt with less holes, the separator is a glass fiber film, and the negative electrode is a structure of a carbon fiber felt loaded Pt/C composite catalyst. The electrolyte contains a high concentration of MnSO4 solution and is charged. When Mn2+ migrates to the surface of the positive carbon fiber, an oxidation reaction generates a layer of MnO2 on the surface of the carbon fiber, and H+ generates a reduction reaction on the surface of the negative electrode to form H2. The process of discharge is just the opposite. MnO2 gains electrons, undergoes a reduction reaction to form soluble Mn2+, returns to the solution to form MnSO4, and H2 oxidizes at the negative electrode to form H+. Wei Chen used the above battery structure to make a battery (as shown in the figure below) and tested the electrochemical performance of the system. In order to reduce the precipitation of O2 on the positive electrode under high voltage in aqueous solution, Wei Chen set the charging voltage at 1.6V. Wei Chen uses 1M MnSO4 as the electrolyte. The first efficiency of the battery is 61%. After more than 10 cycles, the Coulomb efficiency can reach about 91%. Because the Pt catalyst of the negative electrode is more active in the acidic environment, Wei Chen added 0.05M H2SO4 to the solution, which greatly improved the performance of the Mn-H battery and the charging current was increased by three times (1.6V constant voltage charging). Only 85s is needed to complete the charging, the discharge voltage platform has also been significantly improved (about 50mV), and the first efficiency has also been increased to 70%, and in the subsequent cycles, the Coulomb efficiency has reached about 100%. For a power battery, the rate performance is a very critical indicator. Figure b below shows that the Mn-H battery is charged at different current densities after the same charging system (1.6V constant voltage charging to 1mAh/cm2) is charged. The discharge curve shows that after the discharge current density increases from 10 mA/cm2 to 50 and 100 mA/cm2, there is almost no decline in the discharge capacity of the battery, which is consistent with the results of different rate cycles in the following figure c, indicating that Mn- H batteries have very excellent rate performance. More importantly, the Mn-H battery did not experience any decline in its capacity after 10,000 cycles in the case of rapid charging. Although the Mn-H battery has very good rate performance and cycle performance, the utilization efficiency of the electrolyte by the carbon fiber electrode is very low, only about 36%, so the overall energy density of the battery is only 19.6Wh/kg. In order to solve this problem, Wei Chen uses a nanostructured carbon thin film as the electrode, which improves the utilization efficiency of the 4M MnSO4 electrolyte to 74.3%, thereby increasing the energy density of the battery to 139Wh/kg and the volumetric energy reaching 210.6Wh/L. . At the same time, Wei Chen also observed that further increasing the concentration of H2SO4 in the electrolyte can effectively improve the battery's rate performance, reduce the charging time, and increase the discharge voltage platform. However, excessive H2SO4 concentration may cause corrosion problems. Need to be further solved from the perspective of battery structure design. The Mn-H battery is still facing a problem - how to move from the laboratory to the application? The primary goal of solving this problem is to increase the capacity of the Mn-H battery. One measure is to increase the thickness and area of ​​the positive carbon fiber felt. Through this measure, the positive electrode load can be significantly increased, but this leads to Mn-H. The rate of decline in capacity of the battery is accelerated, for example, by thickening the positive carbon fiber felt by a factor of 2, although the capacity of the battery is increased twice, but after 600 cycles, the capacity is reduced to 96.5% of the initial capacity. Another measure is the design of asymmetric positive and negative electrodes. From the working principle of Mn-H batteries, the negative electrode structure mainly catalyzes and does not require storage of H2, so Wei Chen et al. designed the Mn-H battery. It has become a cylindrical structure. By increasing the area of ​​the positive electrode and reducing the area of ​​the negative electrode (as shown in the figure below), the capacity and energy density of the Mn-H battery are significantly improved. At the same time, the amount of Pt/C catalyst is greatly reduced and the The cost of the Mn-H battery. Although this design will reduce the rate capability of the battery to a certain extent (reduction of negative electrode reaction area), this does not prevent the good cycle performance of the battery. As can be seen from the following figure e, the capacity of the battery after 1400 cycles, the capacity The retention rate can still reach 94.2%, fully meeting the demand for power batteries. The Mn-H battery developed by Cui Wei and Wei Chen, etc., is essentially a hybrid battery consisting of a chemical energy storage battery anode and a fuel cell anode, since it does not need to calculate the quality of the H2 cathode. The weight of the battery uses the two-electron reaction of Mn2+/Mn4+ to increase the theoretical capacity of MnO2 to 616 mAh/g. Therefore, although the battery voltage platform is only about 1.3 V, a higher specific energy is still obtained. However, at present, there are still some problems with the battery. First, the carbon fiber felt used for the positive and negative electrodes has a large weight and poor wettability, which reduces the specific energy of the battery and requires a film made of nano-carbon material as an electrode, which pushes up the cost of the battery. In addition, since the battery generates hydrogen gas when it is charged, it needs to use air flow (such as Ar, N2 gas, etc.) to bring the generated H2 out of the battery, and during the discharge process, it needs to supply H2 continuously for the battery, which needs to be outside the battery. Increasing the storage of Ar(N2) and H2 leads to a reduction in the specific energy of the battery system. There is also a hidden problem, in the presence of a small amount of CO, CO2 in H2 (this is the current industry H2 common impurities), may lead to negative catalyst poisoning, affect the battery cycle life, these issues need to In the follow-up battery optimization is resolved. But overall, this is a very creative idea. With good optimization, it can effectively reduce the cost of the battery, which is of great significance for the promotion of large-scale energy storage and electric vehicles. Our range of optical fibres come in either ready-made bundles with various length and diameter fibres so you can create your own bundles. Outdoor fibre optic light bundle,Northern light fibre optic net,Fibre optic bundles for sensory lights Jiangxi Daishing POF Co.,Ltd , https://www.opticfibrelight.com

The ready-made fibre optic bundles come with a connector port so you can easily connect it to the compatible light engines. As different light engines have different port diameters, please consider the compatibility between the light engine and the fibre optic bundle you choose.