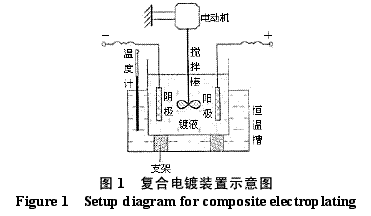
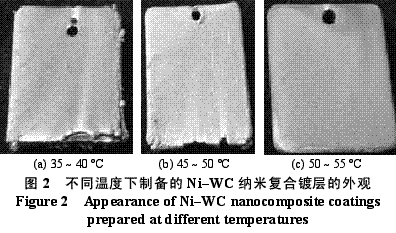
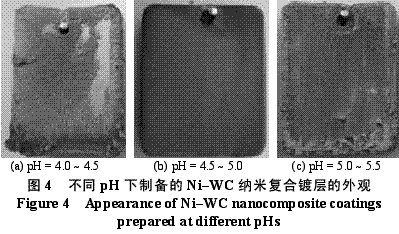
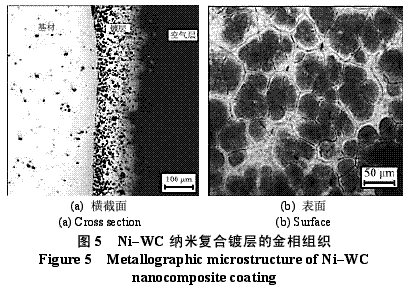
Abstract : Ni-WC nanocomposite coating was prepared by electroplating. The composition of the plating solution was: NiSO4·7H2O250g/L, NiCl2·6H2O30g/L, H3BO330g/L, brightener 0.1g/L, nano WC particles 5~30g/ L, the amount of surfactant and dispersant. The effects of temperature, current density and pH on the appearance of the composite coating were studied. The optimum plating conditions were as follows: temperature 50~55 °C, current density 2~4A/dm2, pH 4.5~5.0. The composite coating prepared under the optimum process conditions has higher microhardness than pure nickel coating and stainless steel, and the abrasion resistance is better than that of stainless steel and pure nickel coating. The corrosion resistance is better than that of stainless steel and is inferior to pure nickel coating. . Key words : nickel; tungsten carbide; nanocomposite coating; microhardness; corrosion resistance; abrasion resistance CLC number : TG174.41; TQ153.12 Document code : A Article ID : 1004-227X (2011) 10-0005-04 1 Introduction Tungsten carbide (WC) has high hardness and high wear resistance. If it can be applied in composite coating materials, it can greatly improve the hardness and wear resistance of materials. Therefore, it is praised as “21st century†by researchers in Europe and America. New water machine materials." As early as the end of the last century, Zhu Longzhang and others studied the composite coating containing micron WC, and found that the hardness of the coating is much higher than other micron composite coatings. The application of nano-scale WC in composite coating materials has also made some progress. Wang Jihui et al. successfully prepared Ni-P-WC nano-particle composite coating. Foreign researchers have used supersonic spraying, plasma spray welding and cold spraying to prepare composite coatings containing nano-WC, but the coating prepared by supersonic spraying has high porosity and oxidation and decay of nanoparticles during spraying. The utilization rate of the nano powder of the plasma spray welding method and the cold spray method is low. In this paper, the Ni-WC nanocomposite coating was prepared by electroplating method, which overcomes the shortcomings of the above preparation methods. The influence of process conditions on the performance of Ni-WC composite coating is studied, in order to obtain a new water machine material with high hardness and good abrasion resistance. . 2. Experiment 2.1 Materials and equipment The 1Cr18Ni9Ti stainless steel plate with 30mm×20mm×5mm was used as the cathode, the electrolytic nickel plate was used as the anode, the ratio of the anode to the anode was 1:2, and the nano-WC particles were obtained by ball milling for 120h, and the average particle diameter was 80nm. The composite plating apparatus is shown in Figure 1. 2.2 Formulation and process 2.2.1 Formulation The formula of ordinary nickel plating solution is: NiSO4·7H2O250g/L, NiCl2·6H2O30g/L, H3BO330g/L, brightener 0.1g/L. Ni-WC composite plating solution is obtained by adding nano-WC powder 5~30g/L and an appropriate amount of surfactant and dispersant to the common nickel plating solution. 2.2.2 Process Cathodic pretreatment process: grinding wheel smoothing - sanding sanding - organic solvent degreasing - washing - hot alkaline washing - washing - weak acid corrosion - washing. Anode pretreatment process: grinding wheel and coarse sandpaper grinding - chemical degreasing - hot water washing - cold water washing - pickling - cold water washing. Before the composite plating, the composite plating solution was placed in a TES-1002 ultrasonic device (Time Ultrasonic Group) and ultrasonically dispersed at 28 kHz for 1 h. The agitation speed during electrodeposition was 150-180 r/min, and the plating time was 4 h. 2.3 Performance test (1) Hardness: The hardness of the coating was tested by HV-5 type small load Vickers hardness tester (Jinan New Era Test Instrument Co., Ltd.), the load was 5 kg, the loading time was 10 s, and the average value of 5 points was the hardness of the coating. (2) Organization structure: The surface structure of the coating was observed by a LEICADFC280 metallographic microscope (Shenzhen Massive Precision Instrument Equipment Co., Ltd.). (3) Corrosion resistance: immersed in a H2SO4 solution having a mass concentration of 20 g/L for 48 hours by a weight loss method, and the corrosion area was 17.0 cm2 (six faces of 30 mm × 20 mm × 5 mm hexahedron). The mass before and after corrosion of the sample was measured by AL204-IC electronic balance (Zhengzhou Boke Instrument Equipment Co., Ltd.), and the corrosion rate was calculated according to formula (1) [6]. (4) Abrasion resistance: stainless steel, pure nickel coating and Ni-WC nanocomposite coating samples with abrading size of 30mm×20mm are simultaneously placed in the aqueous suspension simulating the working conditions of hydraulic mechanical equipment, driven by electric stirring rod The aqueous suspension flows at a speed of 240 r/min. Among them, the aqueous suspension consists of 2 kg of sand and 5 kg of water. The mass before and after the abrasion of the sample was measured by an electronic balance, and the abrasion rate was calculated according to the formula (1). Where v is the rate of corrosion (abrasive) [g/(m2·h)], m1, m2 are the masses before and after corrosion (abrasive) (g), and A is the area of ​​corrosion (abrasive) (m2), t For corrosion (abrasive) time (h). 3. Results and discussion 3.1 Influence of process conditions on composite coating 3.1.1 Temperature Due to the addition of nano-WC powder, the temperature of the plating solution should not be too high, otherwise the oxidation and decay of the nano-powder will be caused, and the low bath temperature should be selected as much as possible under the premise of ensuring the perfect composite coating. Figure 2 shows the appearance of the resulting Ni-WC nanocomposite coating at different temperatures. It can be seen from Fig. 2 that when the bath temperature is 35~40°C and 45~50°C, the nanocomposite coating is peeled off from the surface of the stainless steel substrate; at 50~55°C, the coating does not peel and warp. This is because as the temperature of the plating solution increases, the stress in the plating layer decreases and the elongation increases. Therefore, the ideal plating temperature for Ni-WC nanocomposite plating should be 50~55°C. 3.1.2 Cathode current density Figure 3 shows the appearance of the resulting Ni-WC composite coating at different current densities. As can be seen from Fig. 3, as the cathode current density increases, the burr generated on the surface of the workpiece (especially the edge) increases, and the roughness of the coating increases. In addition, when the coating was observed, when the current density was 1~2 A/dm2, the nano-WC was not deposited on the surface of the substrate, and only the slow deposition of nickel occurred. Therefore, a suitable cathode current density is 2 to 4 A/dm 2 . 3.1.3 pH The pH change of the bath directly affects the performance of the coating. The pH of the conventional nickel bath is usually between 4.0 and 5.5. Figure 4 shows the appearance of the resulting coating at different bath pHs. It can be clearly seen from Fig. 4 that when the pH of the plating solution is 4.0~4.5, the coating layer is unevenly distributed and the surface is rough; when the pH is 5.0~5.5, the plating layer is pinched due to brittleness; when the pH is 4.5~5.0, the surface of the plating layer Even and meticulous, no defects. Therefore, a suitable pH is 4.5 to 5.0. Based on the above analysis, the optimum conditions for the preparation of Ni-WC nanocomposite coatings are: 50~55°C, 2~4A/dm2, pH=4.5~5.0. 3.2 Performance of composite coating 3.2.1 Organizational structure FIG. 5 is a photograph of the metallographic structure of the Ni-WC nanocomposite coating obtained by electroplating for 4 h under the optimal plating process conditions. It can be seen from the metallographic structure of the cross section of the coating (see Fig. 5a) that there is a clear boundary between the composite coating and the substrate, but the two are closely combined. The black particles densely coated in the coating are nano-WC particles, and the white portion is a nickel matrix, indicating that the nano-WC particles are co-deposited with Ni. It can be seen from the metallographic structure on the surface of the coating (Fig. 5b) that nano-WC and Ni have co-deposited to form a relatively large cell structure with obvious grain boundaries, and the center of each grain is black nano. The WC particle aggregation area is surrounded by a white nickel atomic deposit layer. The size of the WC particles in the plating layer is significantly increased compared to after ball milling because the nano WC is agglomerated during the plating process. 3.2.2 Microhardness Microhardness tests were carried out on stainless steel, pure nickel coating and Ni-WC nanocomposite coatings prepared under optimal plating conditions. The results were: 223, 570 and 998 HV, respectively. The microhardness of pure nickel coating is higher than that of stainless steel, while the microhardness of Ni-WC nanocomposite coating is much higher than that of stainless steel and pure nickel coating, indicating that the presence of nano-WC particles in composite coating plays a significant role in dispersion strengthening. . 3.2.3 Corrosion resistance The corrosion resistance of the three samples was compared. The corrosion rates of stainless steel, pure nickel coating and Ni-WC nanocomposite coating were calculated by formula (1): 2.0102, 0.4325 and 0.5543g/(m2·h). . The corrosion rate of stainless steel is the largest, which is 3.6 times and 4.6 times that of Ni-WC nanocomposite coating and pure nickel coating respectively. The corrosion resistance of composite coatings and pure nickel coatings is significantly better than that of stainless steel, but the composite coatings are less corrosion resistant than pure nickel coatings. The reason is that, due to the existence of a large number of Ni/WC interfaces in the composite coating, not only the internal stress is large at the interface, but also the particle is in a high-energy state, and the interface is loose after the corrosion occurs, causing the WC particles to fall off in a cluster, thus forming the formation in Figure 6a. The metallographic structure; as can be seen from Fig. 6b, the surface of the pure nickel coating after corrosion shows uniform pitting, the corrosion occurs uniformly on the surface of the entire coating, and there is no agglomeration of the material, which makes the corrosion of the pure nickel coating lose weight. Less than the nanocomposite coating, it exhibits superior corrosion resistance. 3.2.4 Abrasion resistance The abrasion resistance of stainless steel, pure nickel coating and Ni-WC nanocomposite coating were compared. The abrasion time was 172h. The abrasion rates of the three were 0.9700, 0.7122 and 0.5023g/(m2). · h). The wear resistance of pure nickel coating is better than that of stainless steel. The wear resistance of Ni-WC nanocomposite coating is the best, which is about 2 times and 1.5 times that of stainless steel and pure nickel coating respectively. This shows that the nano-WC particles embedded in the coating improve the abrasion resistance of the coating to some extent. It can be seen from Fig. 5b that the WC particles in the coating layer are obviously agglomerated. Like the corrosion process, the agglomeration of the WC particles occurs during the abrasion process. If the agglomeration problem of the nanoparticles is solved, the Ni-WC nanocomposite coating should be further improved. Abrasion resistance. Based on the above performance analysis, the microhardness of Ni-WC nanocomposite coating is obviously improved compared with stainless steel and pure nickel coating, but the corrosion resistance and abrasion resistance need to be further improved. The key is to overcome the long-term plating process. Agglomeration problems of nano-WC particles. 4 Conclusion (1) The Ni-WC nanocomposite coating was successfully prepared by composite electroplating. The optimum electroplating conditions were as follows: temperature 50~55°C, current density 2~4A/dm2, pH4.5~5.0. (2) The microhardness (998HV) of Ni-WC nanocomposite coating is much higher than that of stainless steel (223HV) and pure nickel coating (570HV). The wear resistance of Ni-WC nanocomposite coating is better than that of stainless steel and pure nickel. However, the corrosion resistance of the composite coating is superior to that of stainless steel and inferior to pure nickel plating. The key to improving the corrosion resistance and abrasion resistance of composite coatings is to solve the problem of agglomeration of nano-WC particles during electroplating. Concerned about surprises Label: Plating Nanoparticles Nanocomposite Nanopowder Corrosion resistance Previous: Filtration heating and cooling of electroplating solution Next: Causes of electroplating plating falling off and its treatment process Reinforced Polypropylene Insulation Reinforced Insulation,Residential Insulation,Insulation Facing Myriad Corporation , http://www.wxfoil.com