(Reporter Zhao Lu) Bisphenol A (BPA) will continue to exist as part of the American diet. The United States Food and Drug Administration (FDA) announced on April 1 that the FDA will not prohibit the use of such chemicals in the packaging of foods and beverages. BPA is a ubiquitous chemical that is believed to be associated with the development of a range of diseases, including heart disease, reproductive problems, behavioral problems, and breast and prostate cancers. Scientists believe that the reason why BPA can cause such a large-scale health impact at lower doses is because it may mimic the effects of estrogen and disrupt the development of the human body, which is particularly serious in infants. However, some industry groups believe that the lack of experimental control, inadequate sample size, or inappropriate test methods has led to conviction in the conclusions of these academic studies, but the United States’s leading canned soup manufacturer has indicated that it BPA will be gradually phased out in the products it produces to prepare for the transformation of the entire industry. In response to a lawsuit filed in August last year by an environmental protection organization from the National Resources Protection Committee in New York, the FDA had to give its own answer. The FDA said: "Some research evidence raises questions about whether BPA is related to a series of health effects, but these studies also have serious problems." The conclusions of the study do not apply to humans, and the rate of human digestion and removal of bisphenol A is also faster than experimental ones. Rats and other animals are much faster. In this statement, the FDA pointed out that the decision is not a "final security conclusion," and the FDA "will continue to support research to test the safety of BPA." In fact, new data from the National Institute of Toxicology Research Laboratory show that BPA dietary exposure in infants is 1/10 of the previous estimate. Since opposition to the ban on BPA, FDA's approach will inevitably anger environmentalists, but some researchers still remain on the sidelines. Scott Belcher, an endocrine-disruption expert at the University of Cincinnati in Ohio, said: “My opinion is that this needs to be very careful. There is still a lot of data to be obtained.†Linda Birnbaum, president of the National Institute of Environmental Health Research (NIEHS), said: "I don't think BPA's story really ended." He said: "Nothing in the new survey results in reducing the amount of BPA Concern and at the same time the most worthy of our attention is the impact of BPA during prenatal development, infant and childhood." The current research on BPA and diabetes has received widespread attention from scientists. Beverly Rubin, a reproductive neuroendocrinologist at Tufts University in Massachusetts, now finds that mice exposed to an appropriate level of BPA require a 3-fold dose of insulin to control glucose levels after a meal. Rubin said: "This is very disturbing." Although most new work reinforces the direct link between BPA and human health issues, a rigorous study yields the opposite result. Because estrogen affects the immune system, researchers hypothesized that low levels of BPA are harmful. To date, however, Paige Lawrence, a toxicologist at the University of Leicester Medical Center in New York, has not found such evidence in mouse studies. Lawrence said: "It's like we have searched through all the stones and wanted to see what they are hiding underneath, but they found nothing. I think this is good news." The National Resources Protection Board petitioned the FDA in 2008 to require the ban on BPA in all food and beverage packaging. After FDA failed to respond, the committee brought it to court. In December last year, the court ruled that the FDA must respond before March this year.
1. Introduction
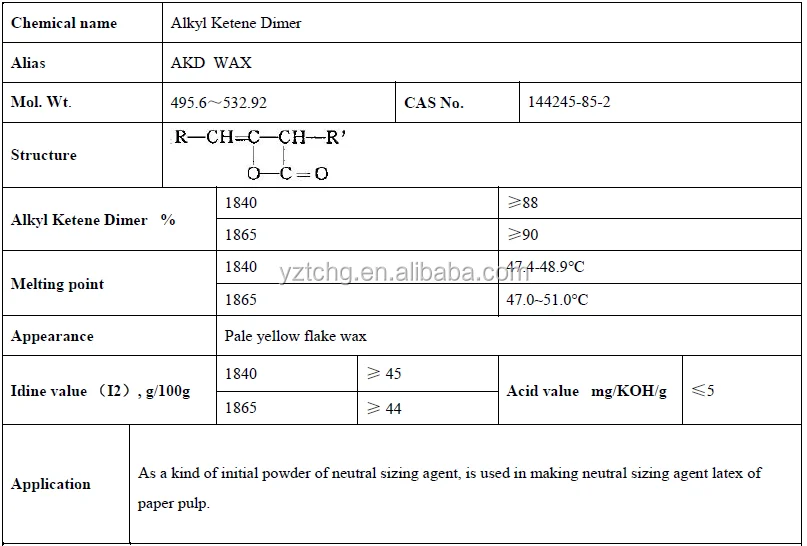
This ADK wax is a very ideal neutral Paper Sizing Agent, it can be used as pulp sizing and surface sizing to make the paper have excellent water resistance. When using this ADK wax, it should be heated to melt, and then mixed with the starch emulsifying agent and protective agent, until the agent emulsify into stable latex
2. Specifications
Appearance
pale yellow waxy solid & flake & block
Purity
≥90%
Melting point
48-50°C
Acid value (mgKOH/g)
2.0 mgKOH/g
FFA free fatty acid
≤2.0%
Iodine value
45- 48.5 giz/100g
Carbon content
C16: 40% C18: 60%
3. Alkyl Ketene Dimer Techinical Data
4. Application of Alkyl Ketene Dimer
As a kind of reactive neutral paper sizing agent, mainly used for copper base paper, copy paper, file paper, paper dictionary and quality writing paper paper in sizing. The pH value can reach about 8, which is called basic sizing, which is widely used at home and abroad. This product can also be used as surface sizing agent.
5. packaging
25KG/ bsg or 500kg/ bag
AKD Wax,Alkyl Ketene Dimer,90% Purity AKD Wax,Alkyl Ketene Dimers AKD Wax Shandong Tiancheng Chemical Co., Ltd. , http://www.tianchengchemical.com
Canada added BPA to the list of toxic substances in the country in 2010, and Canada, the European Union, and 11 states in the United States have banned the use of this chemical in baby bottles.